Abstract
Introduction: Abnormal B-cell receptor (BCR) signaling is a key factor in the pathogenesis of CLL, including in patients who have developed resistance to Bruton tyrosine kinase (BTK) inhibitors. MS-553 is a potent, highly selective, oral, non-covalent inhibitor of protein kinase C (PKC) β, an essential signaling molecule immediately downstream of BTK and PLCγ2 in the BCR pathway. As PKCβ is downstream of both BTK and PLCγ2, inhibition of PKCβ has the potential to treat acquired resistance mutations from either protein (the most common mechanisms of BTKi resistance), unlike second generation BTKi. We report early results from the Phase 1/2 dose escalation and expansion study in patients with CLL/SLL treated with MS-553 (NCT03492125).
Methods: The primary objective of the study is to evaluate the safety and tolerability of oral daily continuous MS-553 as monotherapy (Cohort A) in relapsed and refractory patients with CLL/SLL, and in combination with acalabrutinib (Cohort B), or with venetoclax plus an anti-CD20 antibody (Cohort C) in earlier lines of therapy. Secondary objectives include assessment of the pharmacokinetic profile, pharmacodynamic activity, and evidence of anti-tumor activity following MS-553 treatment. Dose expansion is underway in cohort A. Dose escalation to determine the recommended Phase 2 dose in the combination cohorts has begun using a conventional 3+3 design. Treatment emergent adverse events (TEAEs) were assessed per NCI CTCAE v.5.0. Tumor responses were evaluated according to IWCLL 2008 guidelines.
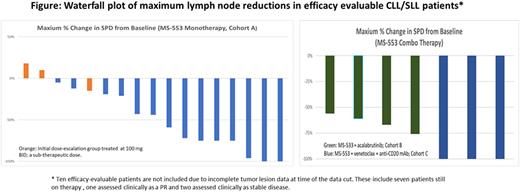
Results: As of June 20, 2022, a total of 45 patients (median age 69; range 38-82) have been treated with at least one dose of therapy. The median number of prior therapies was 4 (range 1-12) for Cohort A (n= 37), 2 (range 0-2) for Cohort B (n=4) and 0.5 (range 0-2) for Cohort C (n=4). Overall, 72% were IGHV unmutated; 44% had del(17p) or TP53 mutations. A total of 76% had previously been treated with at least one or more BTKi. For Cohort A, 92% had previously received BTKi, 51% had received venetoclax, and 41% had received both BTKi and venetoclax. To date, one dose limiting toxicity (Grade 3 Acute Kidney Injury) has been observed in the highest dose group, but the MTD has not been reached. The majority of the drug-related TEAEs were grade 1 or 2. The most common (>10%) were nausea (36%); diarrhea (29%); fatigue (18%); vomiting, anemia, and abdominal pain (13% each); and anorexia (11%). Drug-related TEAEs ≥ grade 3 include neutropenia (n=2) and one case each of nausea, diarrhea, fatigue, weight loss, lymphocyte increase, hyponatremia, acute kidney injury, and generalized muscle weakness. No drug-related atrial fibrillation or bleeding has been reported. For efficacy evaluable patients in Cohort A, 50% (10/20) of patients exhibited a partial response and 50% stable disease with the median duration of therapy of 6.0 and 4.1 months respectively. In cohort B and C, all patients responded (B, 4/4 PRs; C, 2/3 PRs and 1/3 CR) and all remained on therapy at time of data cut. Of note, responses occurred in several ultra-high-risk patients such as those with BTK/PLCG2/TP53 triple mutations, with relapse after BTKi and venetoclax treatment, and having relapsed after both covalent and non-covalent BTKi. For the combination Cohorts B and C, all participants have shown partial or complete responses and are continuing therapy as of the data lock. While still in the dose escalation phase of the study, the median time on therapy for Cohort B is 5.6 months and for Cohort C is 6 months.
Conclusion: MS-553 has been generally well tolerated with initial anti-tumor activity seen in this heavily pretreated population. Half of the evaluable patients have shown partial responses. The combination cohorts have shown good initial safety and tolerability.
Disclosures
Blachly:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; INNATE Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; MingSight Pharmaceuticals: Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees. Stephens:Arqule: Research Funding; TG Therapeutics: Consultancy; Epizyme: Consultancy; Newave: Research Funding; Celgene: Consultancy; Genentech: Consultancy; Lilly: Consultancy; AstraZeneca: Consultancy; CSL Behring: Consultancy; Beigene: Consultancy; Mingsight: Research Funding; Novartis: Research Funding; AbbVie: Consultancy; JUNO: Research Funding; Karyopharm: Research Funding; Acerta: Research Funding. Ye:BMS: Consultancy; Ascentage: Research Funding; sanofi: Research Funding; Karyopharm: Research Funding; Portola: Research Funding; MingSight: Research Funding; Nektar: Research Funding; Janssen: Consultancy, Research Funding; GSK: Research Funding; Regeneron: Research Funding; Genmab: Research Funding. Lamanna:Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZenenca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Loxo Oncology/Eli Lilly and Company: Research Funding; Mingsight: Research Funding; Octapharma: Research Funding; Oncternal: Research Funding; TG Therapeutics: Research Funding. Jain:AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Servier Pharmaceuticals LLC: Research Funding; ADC Therapeutics: Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Cellectis: Honoraria, Research Funding; Incyte Corporation: Research Funding; Pfizer: Research Funding; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Mingsight: Research Funding; Takeda: Research Funding; Medisix: Research Funding; Loxo Oncology: Research Funding; Novalgen: Research Funding; Dialectic Therapeutics: Research Funding; Newave: Research Funding; TransThera Sciences: Research Funding; Beigene: Honoraria; Cellectis: Honoraria, Research Funding; TG Therapeutics: Honoraria; MEI Pharma: Honoraria; Ipsen: Honoraria; CareDx: Honoraria. Niesman:MingSight Pharmaceuticals: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Zhang:MingSight Pharmaceuticals: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties. Woyach:Karyopharm Therapeutics: Research Funding; AbbVie: Consultancy, Research Funding; Loxo@Lilly: Research Funding; MorphoSys: Consultancy, Research Funding; Schrodinger: Research Funding; ArQule: Consultancy; AstraZeneca: Consultancy; BeiGene: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Newave: Consultancy; Pharmacyclics: Consultancy.
OffLabel Disclosure:
MS-553 is not yet FDA approved for any indication and is in trial for the treatment of CLL among other indications.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal